I-Rezdiffra (resmetirom) igunyazwe yi-FDA yase-USA ukuze zelashwe abantu abadala abane-noncirrhotic non-alcoholic steatohepatitis (NASH) enezibazi zesibindi ezimaphakathi kuya kweziphambili (fibrosis), ukuthi zisetshenziswe kanye nokudla nokuzivocavoca.
Kuze kube manje, iziguli ezine-noncirrhotic non-alcoholic steatohepatitis (NASH) nazo ezinezibazi zesibindi eziphawulekayo bezingenayo imithi engakwazi ukubhekana ngqo nazo. ukulimala kwesibindi. Ama-FDA's ukugunyazwa kwe-Rezdiffra kuzohlinzeka, ngokokuqala ngqa, a ukwelashwa inketho yalezi ziguli, ngaphezu kokudla nokuzivocavoca.
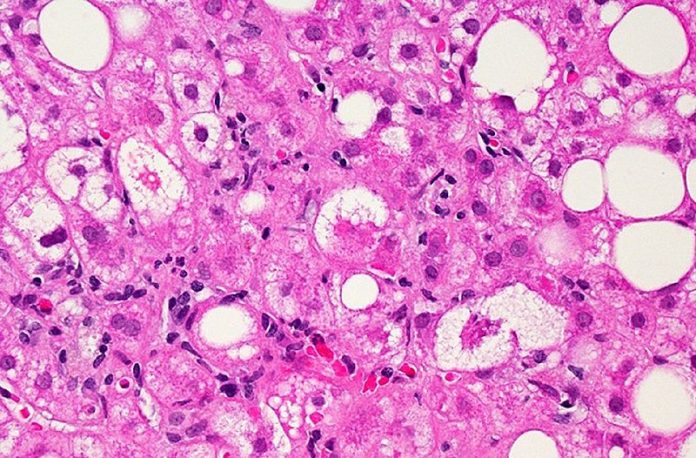
I-NASH ingumphumela wokuqhubekela phambili kwamafutha angewona utshwala isibindi isifo lapho isibindi ukuvuvukala, ngokuhamba kwesikhathi, kungaholela esikhumbeni sesibindi kanye nokungasebenzi kahle kwesibindi. I-NASH ivame ukuhlotshaniswa nezinye izinkinga zempilo ezifana nomfutho wegazi ophakeme kanye nesifo sikashukela sohlobo 2. Okungenani ukulinganisa okukodwa, cishe abantu abayizigidi ezingu-6-8 e-US bane-NASH enezibazi zesibindi ezimaphakathi kuya kwezithuthukile, nalelo nani okulindeleke ukuba likhule.
I-Rezdiffra iyi-activator eyingxenye ye-receptor ye-hormone yegilo; ukusebenza kwalesi receptor nge-Rezdiffra esibindini kunciphisa ukuqoqwa kwamafutha esibindi.
Ukuphepha nokusebenza ngempumelelo kwe-Rezdiffra
Ukuphepha nokusebenza ngempumelelo kwe-Rezdiffra kwahlaziywa ngokususelwa ekuhlaziyweni kwesiphetho esithathwe esikhundleni senyanga ye-12 ocwaningweni olulawulwa yi-placebo lwezinyanga ezingama-54, olungahleliwe, oluyimpumputhe kabili. Iphoyinti lokugcina lokuthatha indawo lilinganise ubukhulu be isibindi ukuvuvukala kanye nezibazi. Umxhasi kudingeka enze ucwaningo lwangemva kokugunyazwa ukuze aqinisekise futhi achaze izinzuzo zomtholampilo ze-Rezdiffra, ezizokwenziwa ngokuqeda ucwaningo olufanayo lwezinyanga ezingama-54, olusaqhubeka. Ukubhalisa ocwaningweni, iziguli kwakudingeka zibe ne- isibindi i-biopsy ebonisa ukuvuvukala ngenxa ye-NASH emaphakathi noma ethuthukile isibindi izibazi. Esivivinyweni, izihloko ze-888 zabelwe ngokungahleliwe ukuze zithole okukodwa kwalokhu okulandelayo: i-placebo (izifundo ze-294); 80 milligrams of Rezdiffra (298 izifundo); noma 100 milligrams of Rezdiffra (296 izifundo); kanye ngosuku, ngaphezu kokunakekelwa okujwayelekile kwe-NASH, okuhlanganisa ukwelulekwa ngokudla okunempilo nokuzivocavoca umzimba.
Ezinyangeni eziyi-12, ama-biopsies esibindi abonisa ukuthi ingxenye enkulu yezifundo ezalashwa nge-Rezdiffra zazuza ukulungiswa kwe-NASH noma ukuthuthukiswa kwezibazi zesibindi uma kuqhathaniswa nalabo abathole i-placebo. Isamba esingama-26% kuya ku-27% wezifundo ezithole ama-milligrams angu-80 e-Rezdiffra kanye nama-24% kuya ku-36% wezifundo ezithole amamiligremu angu-100 e-Rezdiffra ahlangabezane nesinqumo se-NASH futhi akukho ukuwohloka kokulimala kwesibindi, uma kuqhathaniswa no-9% kuya ku-13% walabo abaye uthole i-placebo kanye nokwelulekwa ngokudla kanye nokuzivocavoca. Uhlu lwezimpendulo lubonisa ukufundwa kwama-pathologists ahlukene. Ngaphezu kwalokho, inani lama-23% ezifundo ezithole amamiligremu angama-80 e-Rezdiffra kanye nama-24% kuya ku-28% wezifundo ezithole amamiligremu ayi-100 e-Rezdiffra bathole intuthuko isibindi izibazi futhi akukho ukuwohloka kwe-NASH, uma kuqhathaniswa no-13% kuya ku-15% walabo abathole i-placebo, kuye ngokufundwa kwe-pathologist ngayinye. Ukuboniswa kwalezi zinguquko engxenyeni yeziguli ngemva nje konyaka owodwa wokwelashwa kuyaphawuleka, njengoba i- isifo ngokuvamile iqhubeka kancane neziguli eziningi ezithatha iminyaka noma ngisho namashumi eminyaka ukubonisa ukuqhubeka.
Imiphumela emibi ye-Rezdiffra
Imiphumela evame kakhulu ye-Rezdiffra yayihlanganisa isifo sohudo nesicanucanu. I-Rezdiffra iza nezixwayiso ezithile nezinyathelo zokuphepha, njengobuthi besibindi obubangelwa izidakamizwa kanye nemiphumela engemihle ehlobene nenyongo.
Ukusetshenziswa kwe-Rezdiffra kufanele kugwenywe ezigulini ezine-cirrhosis eboshiwe. Iziguli kufanele ziyeke ukusebenzisa i-Rezdiffra uma ziba nezimpawu noma izimpawu zokuwohloka isibindi ukusebenza ngenkathi ukwelashwa kwe-Rezdiffra.
Ukusebenzisana kwezidakamizwa kwe-Rezdiffra
Ukusebenzisa i-Rezdiffra ngasikhathi sinye nezinye izidakamizwa ezithile, ikakhulukazi ama-statins okwehlisa i-cholesterol, kungaholela ekusebenzisaneni kwezidakamizwa okungaba okubalulekile. Abahlinzeki bezempilo kufanele babhekisele olwazini olugcwele oluchazayo ukuze bathole ulwazi olwengeziwe mayelana nalokhu kusebenzisana kwezidakamizwa okungaba okubalulekile ne-Rezdiffra, umthamo onconyiwe kanye nezinguquko zokuphatha.
The FDA igunyazwe i-Rezdiffra ngaphansi kwendlela yokugunyazwa esheshisiwe, evumela ukugunyazwa kwangaphambi kwesikhathi kwemithi elapha izimo ezibucayi futhi ibhekane nesidingo sezokwelapha esingahlangatshezwana naye, ngokusekelwe endaweni yokwelashwa ebambele noma emaphakathi okungenzeka ibikezele inzuzo yomtholampilo. Ucwaningo oludingekayo olushiwo ngenhla lwezinyanga ezingama-54, oluqhubekayo, luzohlola inzuzo yomtholampilo ngemva kwezinyanga ezingu-54 zokwelashwa kwe-Rezdiffra.
U-Rezdiffra uthole ukuqokwa kwe-Breakthrough Therapy, i-Fast Track kanye ne-Priority Review yalesi sibonakaliso.
The FDA inikeze imvume ye-Rezdiffra kwaMadrigal Pharmaceuticals.
***
Source:
I-FDA 2024. Ukukhishwa Kwezindaba - I-FDA Igunyaza Ukwelashwa Kokuqala Kweziguli Ezinezibazi Zesibindi Ngenxa Yesifo Sesibindi Esinamafutha. Kuthunyelwe ngomhla ka-14 Mashi 2024. Itholakala e- https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-liver-scarring-due-fatty-liver-disease
***